
It was ~50 years later that Italian chemist Stanislao Cannizzaro resurrected Avogadro’s ideas and showed how atomic weights could be calculated unambiguously. But how does one obtain an absolute weight of an atom? In 1811, Italian physicist and mathematician Amedeo Avogadro proposed an idea to calculate atomic weights from gases, but his hypothesis was not widely accepted. A key advance was Dalton’s assignment of relative weights to the atoms of elements. Such is the story of the periodic table.Īmong the critical events that led to the discovery of the periodic table was the emergence of atomic theory that had been initially proposed in 1808 by John Dalton, a British tutor and schoolteacher. But, sometimes, scientific discoveries are made simultaneously by different researchers. But does Mendeleev deserve all the credit? Scientific discoveries rarely arise in isolation rather contributions from researchers over many years lead to a general picture that eventually emerges.

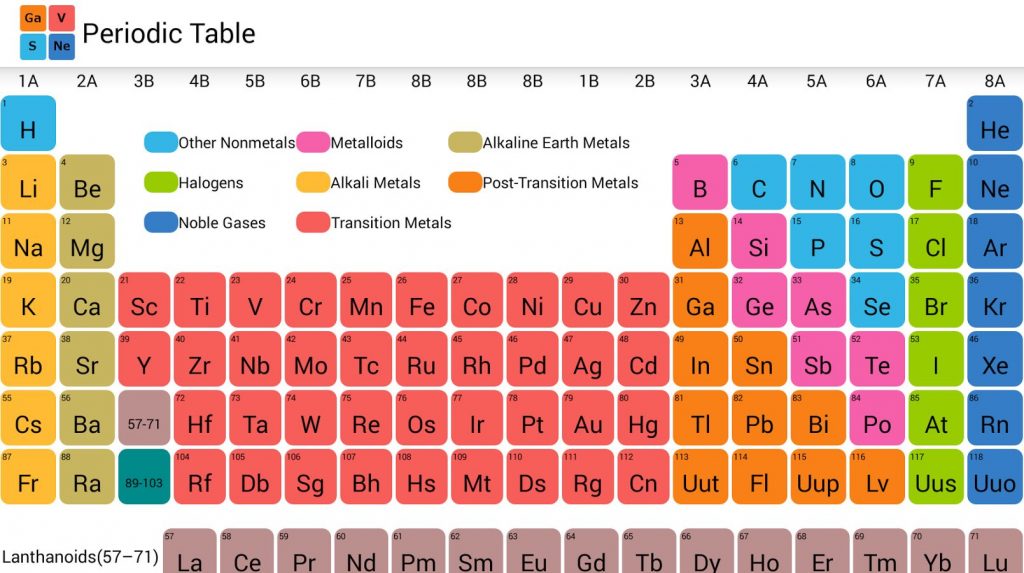
To celebrate the 150th-anniversary of this great achievement, the United Nations and UNESCO declared 2019 to be the International Year of the Periodic Table of Elements.

His table expresses the periodic law: elements arranged according to the size of their atomic weights show periodic properties. So how did this “Rosetta Stone of Nature” originate? Most likely, you will answer Dmitri Mendeleev, the Russian chemist who in 1869 published a version of the periodic table that we recognize today. The periodic table of chemical elements is one of the most significant achievements in science because it arranges the 118 known elements in a deceptively simple pattern that reveals their properties.


 0 kommentar(er)
0 kommentar(er)
